Every day, we use soap—often without considering the fascinating science behind this humble household staple. As a scientist, I find the chemistry of soap both elegant and essential, bridging the worlds of biology, chemistry, and everyday life. In this article, let’s unravel the chemical magic that turns fatty substances and alkali into a substance that keeps us clean and healthy.
The Building Blocks: Fats and Alkalis
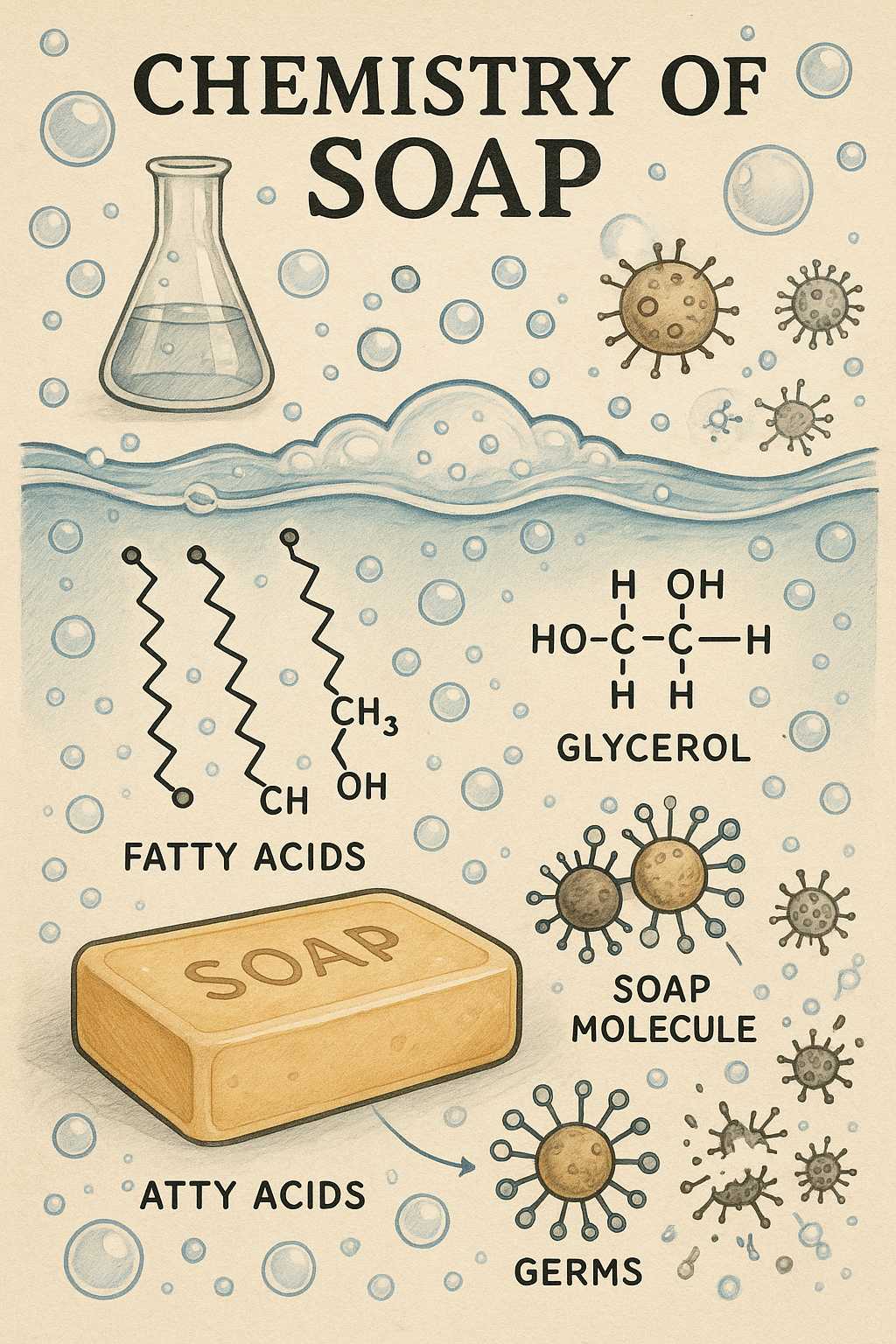
Soap is made through a process called saponification. This reaction combines fats or oils (triglycerides) with a strong base, typically sodium hydroxide (lye). When these ingredients react, the triglycerides break apart, forming glycerol and soap molecules (sodium or potassium salts of fatty acids).
The overall reaction looks like this:
Fat + Strong Base → Soap + Glycerol
This simple equation paved the way for soapmaking—an art practiced for thousands of years.
How Soap Cleans: Breaking Down the Science
The cleaning power of soap comes from its amphiphilic structure: each soap molecule has a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. The tail loves grease and oil, while the head clings to water.
When you wash your hands with soap and water, the molecules surround grease, oil, and dirt, forming tiny spheres called micelles. The hydrophobic tails encapsulate the dirt, while the hydrophilic heads face outward into the surrounding water. When rinsed, these micelles—and whatever they’ve trapped—are washed away, leaving your hands clean.
Soap and Germs: More Than Just Cleaning
Besides removing dirt and oil, soap is also effective at disrupting the lipid membranes of many microorganisms, including certain viruses and bacteria. This is especially important for defending against illnesses such as the flu or COVID-19. The soap interferes with the structural integrity of these pathogens, rendering them inactive and washing them away.
The Science of Bubbles
Ever wondered why soap lathers into bubbles? The micelles also help trap air, forming stable bubbles. While bubbles themselves don’t aid in cleaning, they offer a satisfying tactile experience and help signal where soap remains on your skin.
Beyond Traditional Soap: Detergents and Modern Formulations
Modern cleaning agents—detergents—are chemically related to soap but often have different, more complex structures designed to work better in hard water and across a range of cleaning tasks. Nonetheless, the fundamental chemistry remains the same: amphiphilic molecules breaking up grease and carrying it away.
Final Thoughts
The next time you lather up, take a moment to appreciate the chemistry at your fingertips. Soap is much more than a household necessity—it’s a marvel of simple, elegant science, protecting us by harnessing fundamental principles of chemistry.
—Carl

Leave a Reply